
Click here:point_up_2:to get an answer to your question :writing_hand:1 223 g ethanol is dissolved in 36 g water find mole fraction of ethanol2
Click here👆to get an answer to your question ✍️ -1- 2 23 g ethanol is dissolved in 36 g water- Find mole fraction of ethanol -2- 0-5 -3- 0-2 -4- 0-8 TIN

Calculate the molarity of a solution of ethanol in water in which

23g ethanol is dissolved in 36g water.find mole fraction of

Calculate mole fraction of ethyl alcohol and water in a solution containing 46 g ethyl and 36g w
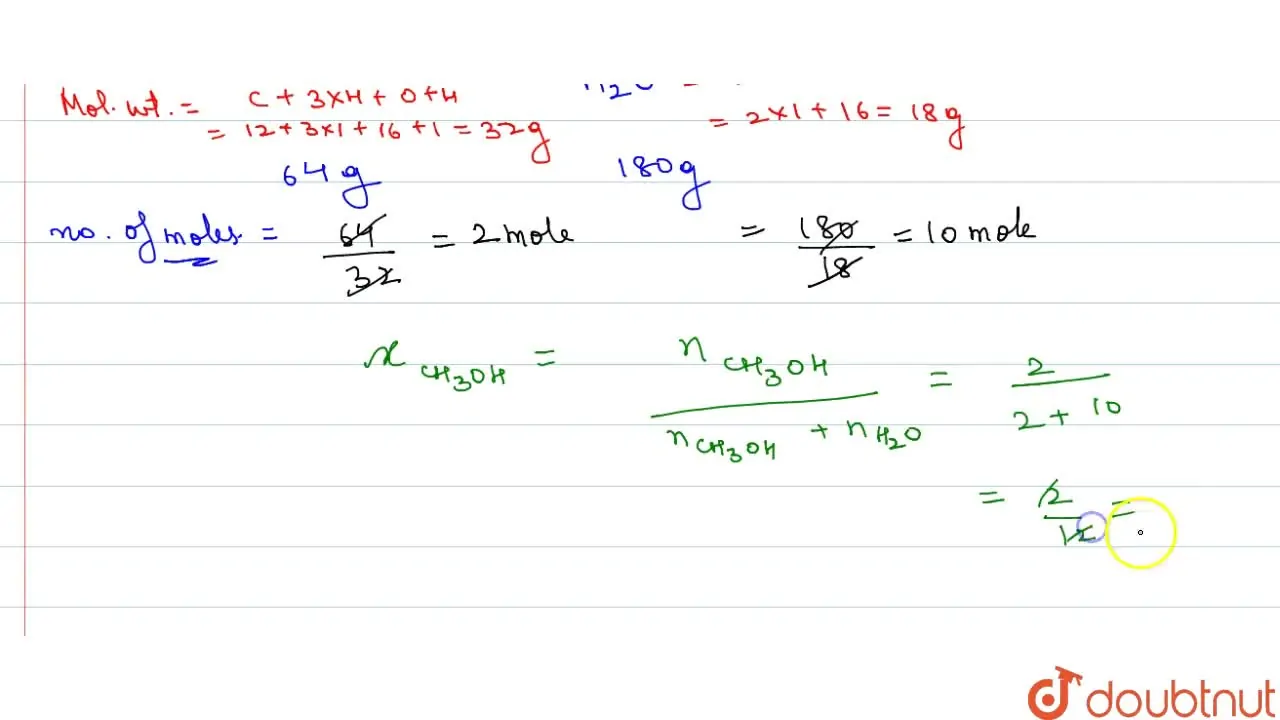
A solution is prepared by adding 64 g of CH(3)OH to 180 g of water. Ca

23 g ethanol is dissolved in 36 g water. Find mole fraction of

SOLVED: Ethanol-water mixture has 46% ethanol (weight/solution
A mixture has 18 g water and 414 g ethanol. The mole fraction of

Calculate mole fraction of ethyl alcohol and water in a solution

Mole fraction (x3) solubility of caffeine (3) in some {cosolvent

UF ethanol is dissolved in 36 g water Find mole fraction of
In a certain solution of ethanol and water, the mole of fraction
What is the mole fraction of substances in a solution containing











