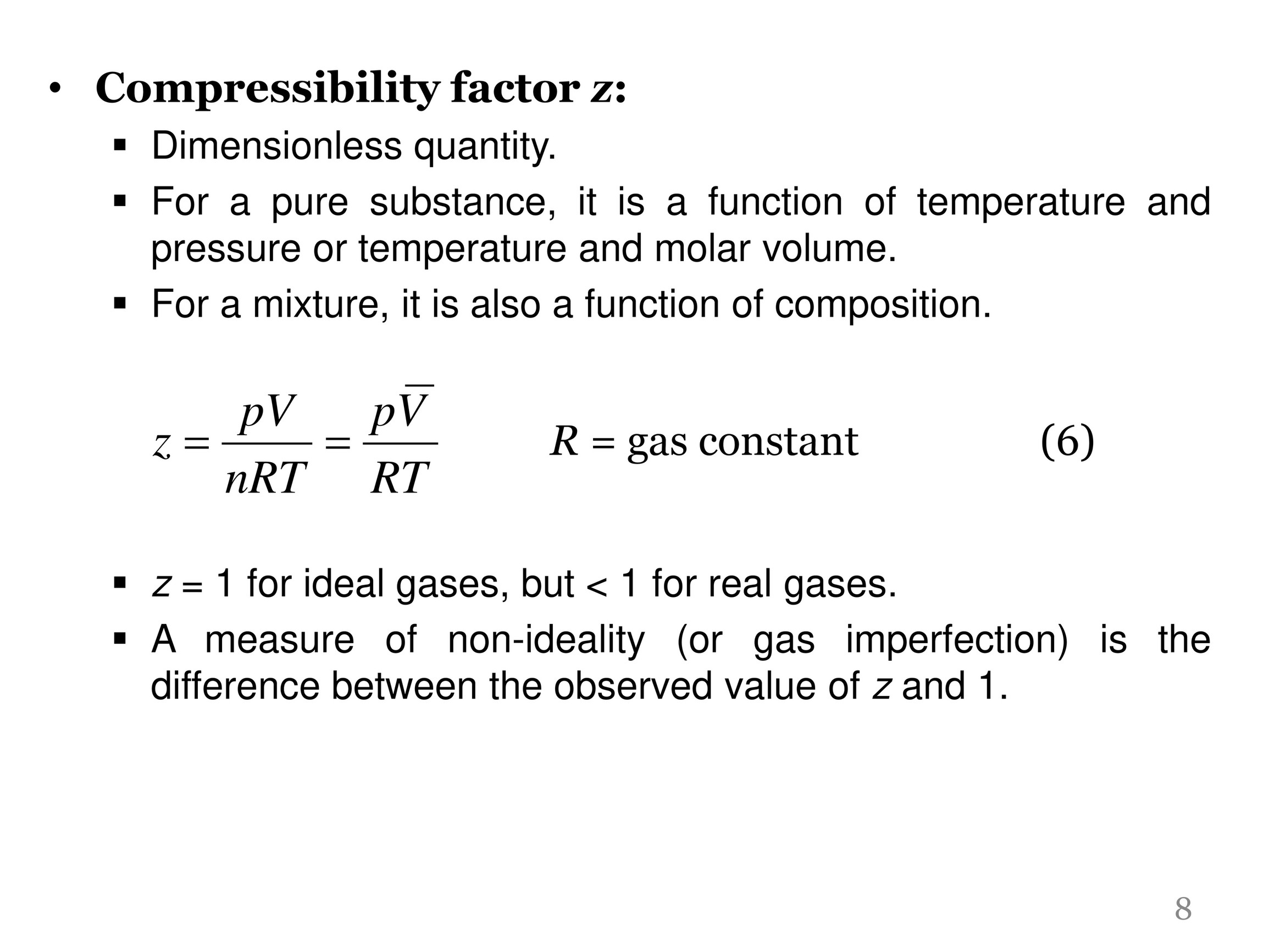
Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT

Click here:point_up_2:to get an answer to your question :writing_hand:compressibility factor z of a gas is given as z frac pv nrt
Click here👆to get an answer to your question ✍️ Compressibility factor- Z of a gas is given as Z- frac - pV - nRT - -i- What is the value of Z an ideal gas-ii- For real gas what will be the effect on value of Z above Boyle temperature

Compressibility Factor - an overview

Figure . Compressibility factor Z = PV/NkT of the SW fluid plotted

Compressibility factor - Wikipedia
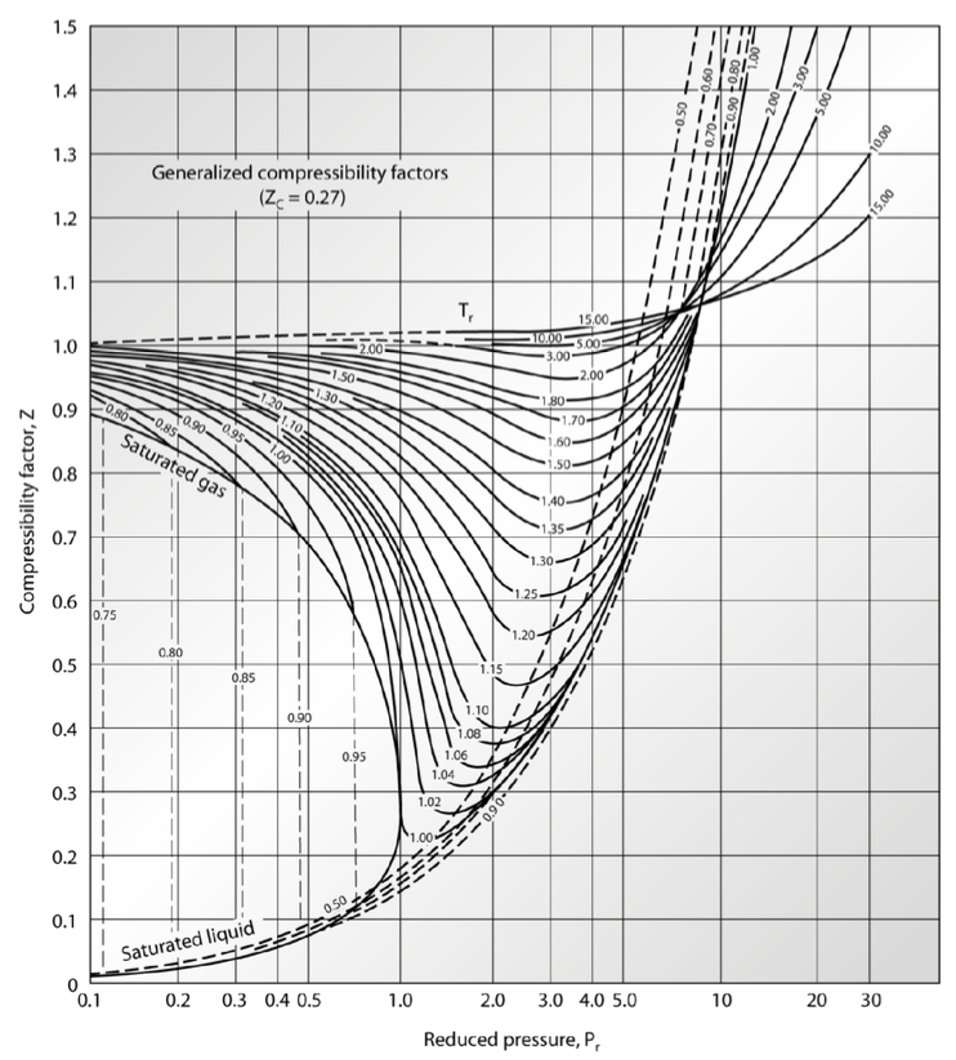
My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created with Publitas.com

The given graph represents the variation of compressibility factor Z vs P for three gases A, B and C.Identify the incorrect statements.

SOLVED: Derive the mathematical expression expressing the compressibility factor Z of a real gas depending on the reduced variables; Explain in detail how the volume of the actual gas at a given

Compressibility factor (gases) - Knowino
What is the value of compressibility factor for a non-ideal gas? - Quora

1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts
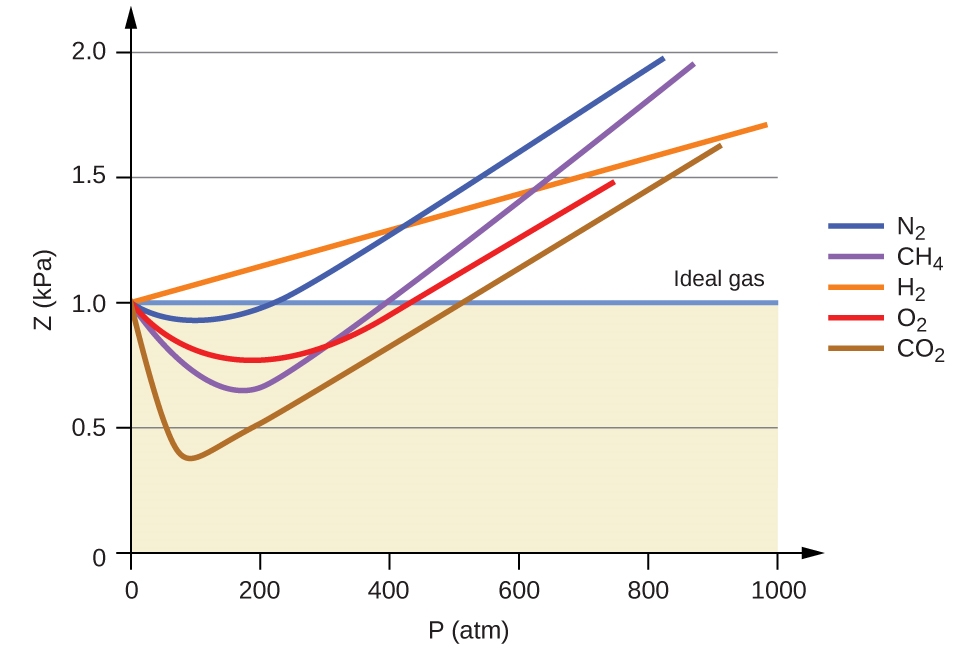
2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange
The given graph represent the variations of compressibility factor (z) = pV/ nRT versus p, - Sarthaks eConnect

Compressibility factor of n-decane vapor (upper graph) and of ethylene
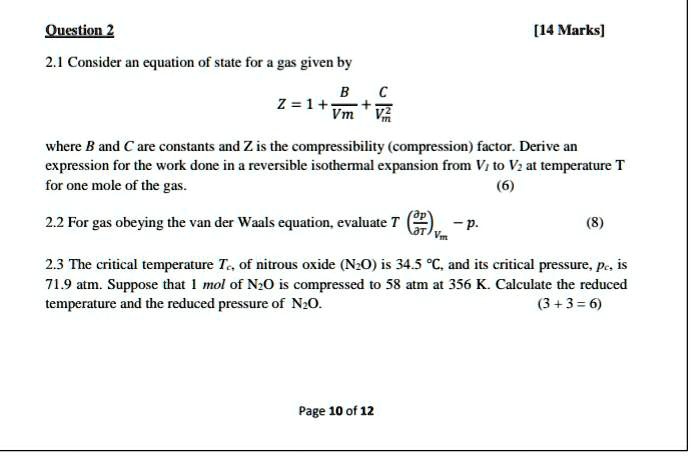
SOLVED: Qussion 2 [14 Marks] 2.1 Consider an equation of state for gas given by 2 =1+ Vm Va where B and € are constants and Z is the compressibility ( compression) factor.