
An ideal gas initially at a state (P1,V1) is allowed to expand isothermally to a state (P2, V2).
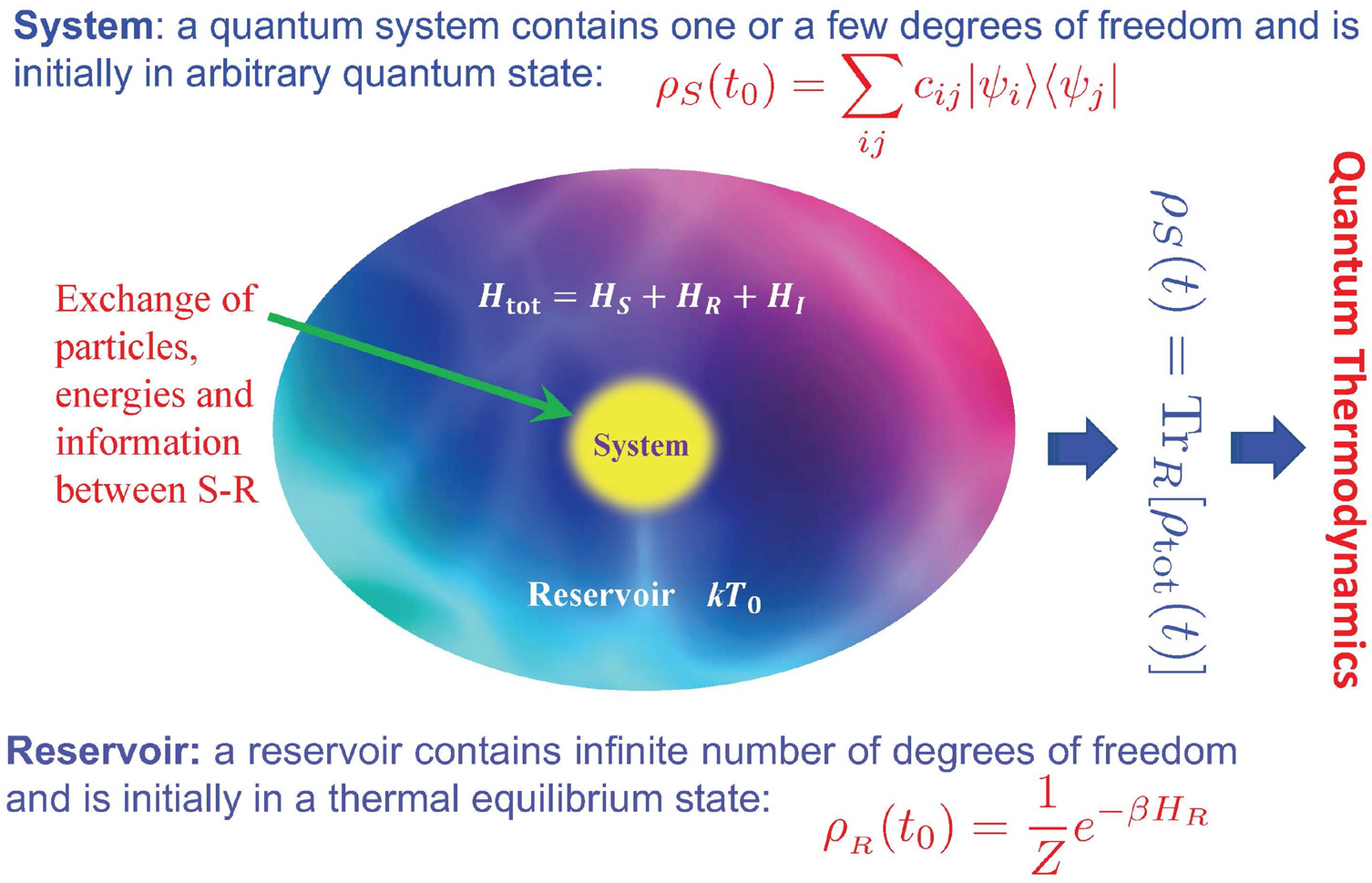
Quantum thermodynamics of single particle systems

Thermodynamics problems
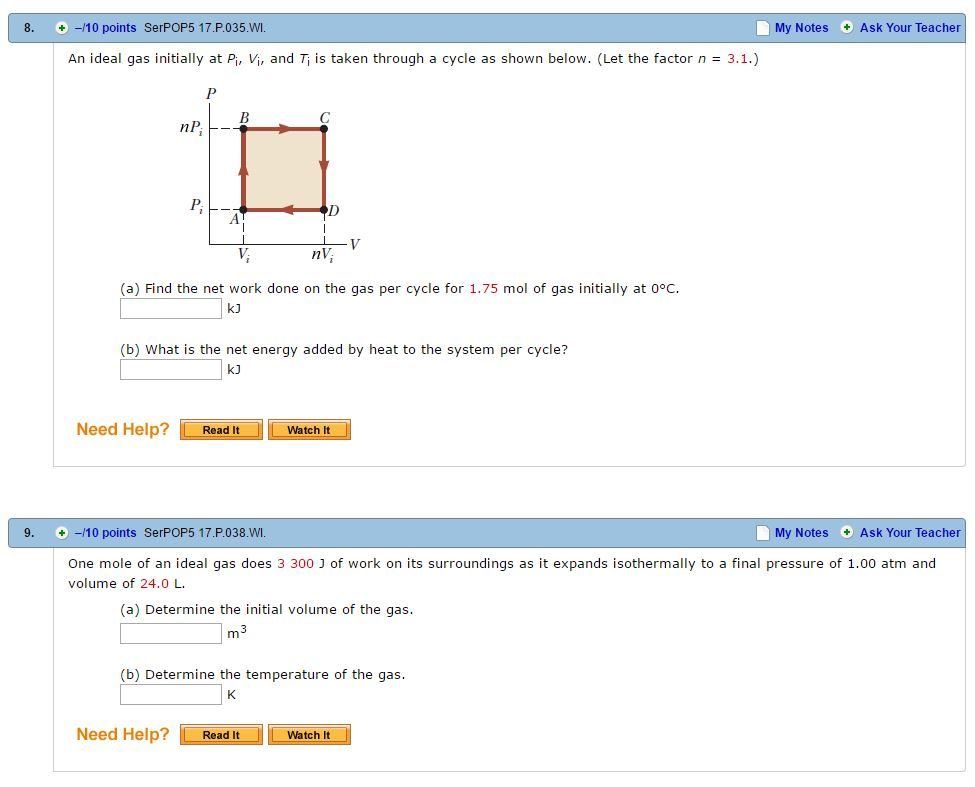
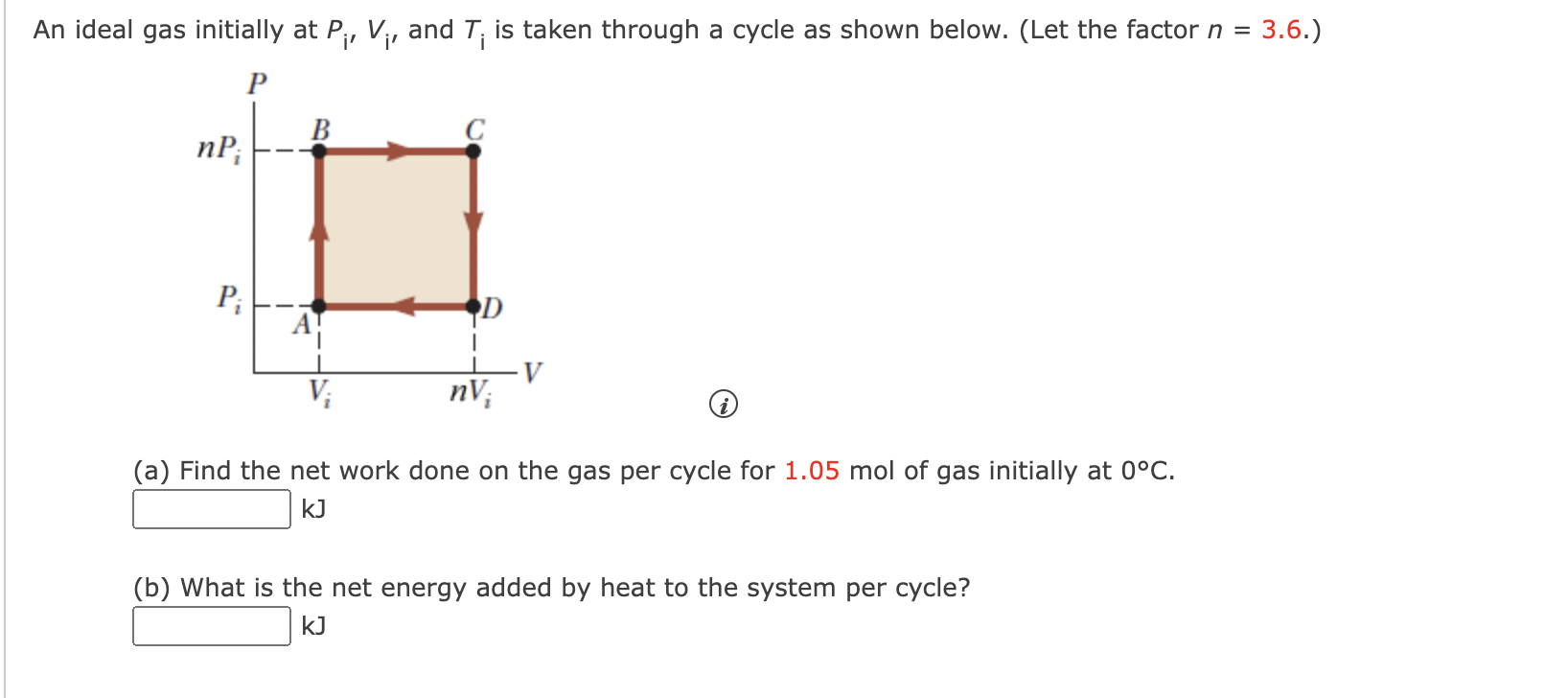
Solved An ideal gas initially at P_i, V_i, and T_i is taken
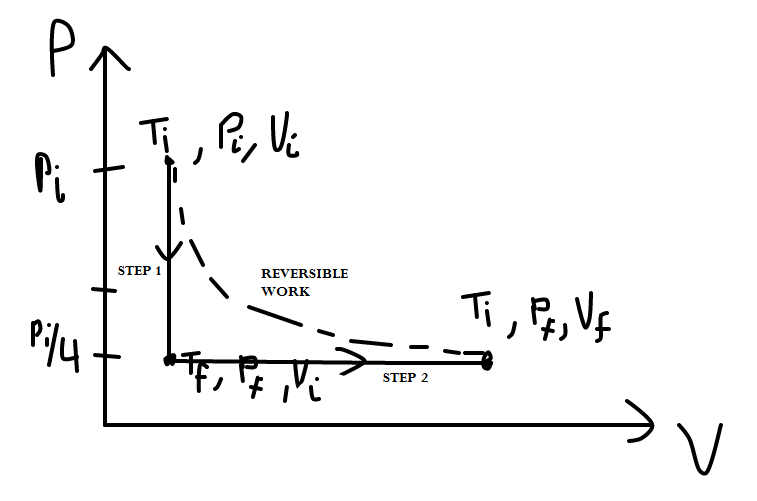
One mol of an ideal gas, initially at 300 K, is cooled at constant V so that P_f is 1/4 P_i. Then the gas expands at constant P until it reaches T_i

What is the Maxwell-Boltzmann distribution? (article)
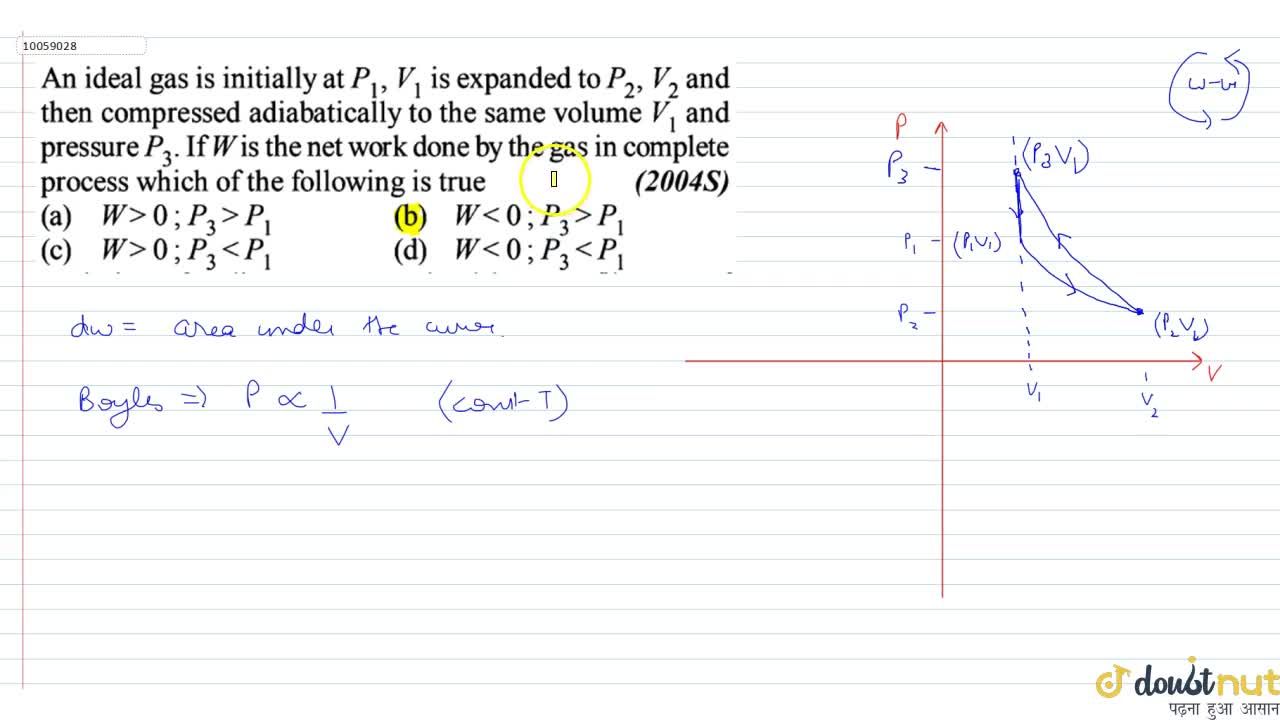
An ideal gas is initially at P1,V1 is expands to P2,V2 and then compre
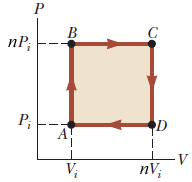
Solved An ideal gas initially at Pi, Vi, and Ti is taken

An ideal gas at a given state expands to a fixed final volume first at constant pressure and then at

SOLVED: An ideal gas initially at Pi, V, and T;is taken through a cycle as shown in Figure What is the net energy added by heat to the gas per cycle for

Solved An ideal gas initially at Pi, V;, and T; is taken

An ideal gas is initially P_1, V_1 is expanded to P_2, V_2 and then compressed adiabatically to the same volume V_1 and pressure P_3. If W is the net work done by
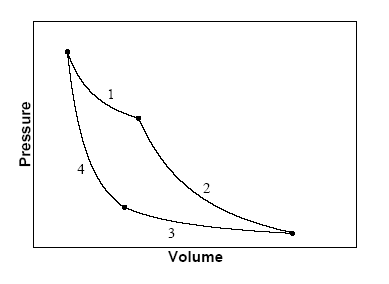
entropy







