physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

In the above graph,the minima of the curve for methane is more than that of nitrogen. Also, for a given value of pressure, the value of $Z$ for methane is less than that of nitrogen. They seem to m

Cold fusion - Wikipedia
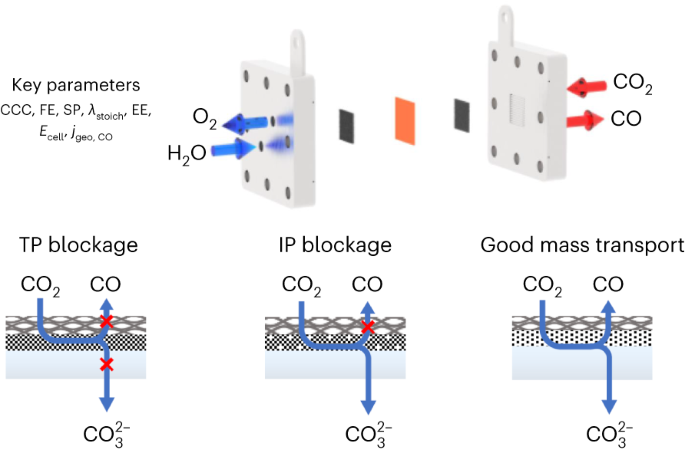
Design and diagnosis of high-performance CO2-to-CO electrolyzer
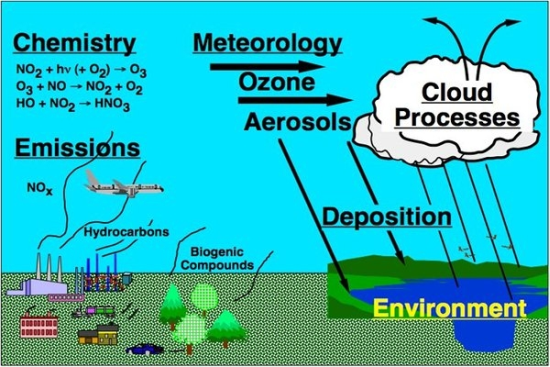
Atmosphere, Free Full-Text

Van der Waals equation - Wikipedia
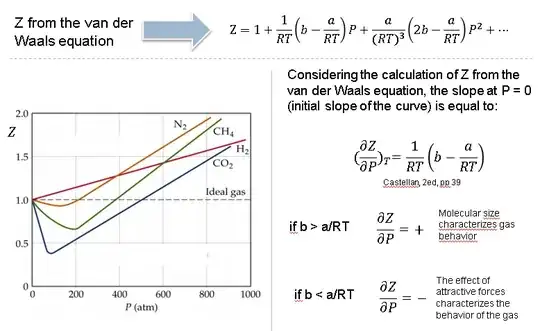
Why do some gases have lower value of Z for a particular pressure
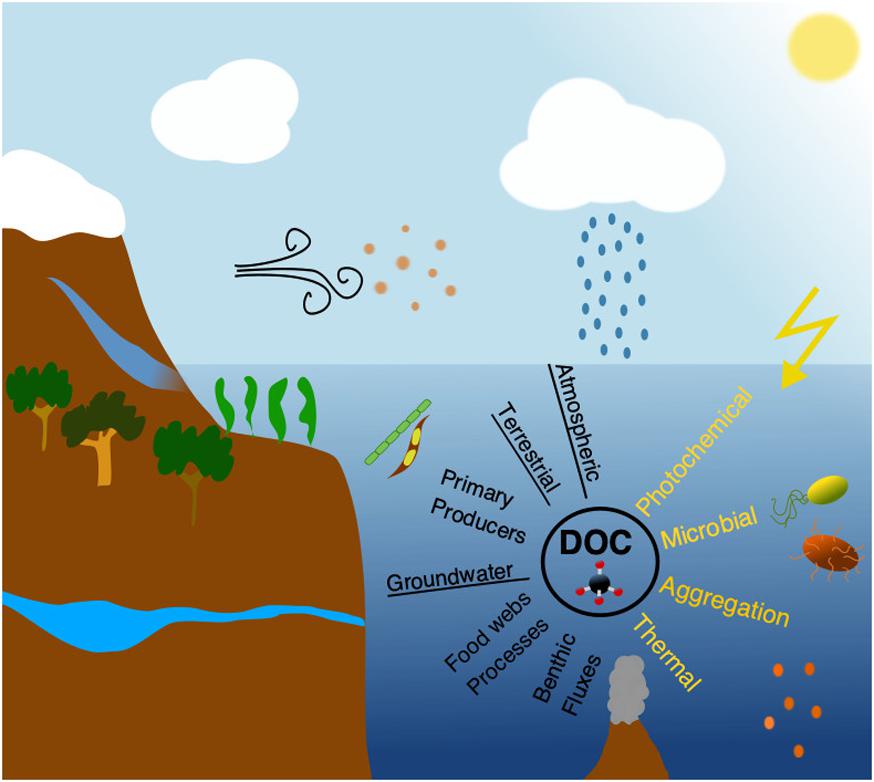
Frontiers Impacts of Global Change on Ocean Dissolved Organic
Pressure - Wikipedia
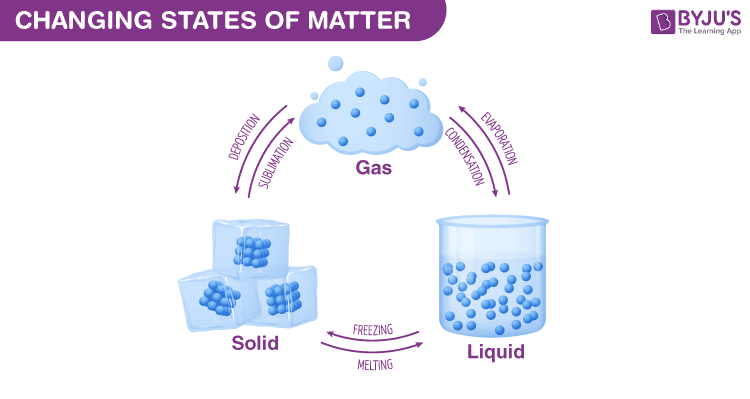
Changing States Of Matter - Solid, Liquid And Gas

Minerals, Free Full-Text

Non-Ideal Gas Behavior Chemistry: Atoms First

Partial Pressure- Formula, Dalton's Law, Mixture of Ideal Gas
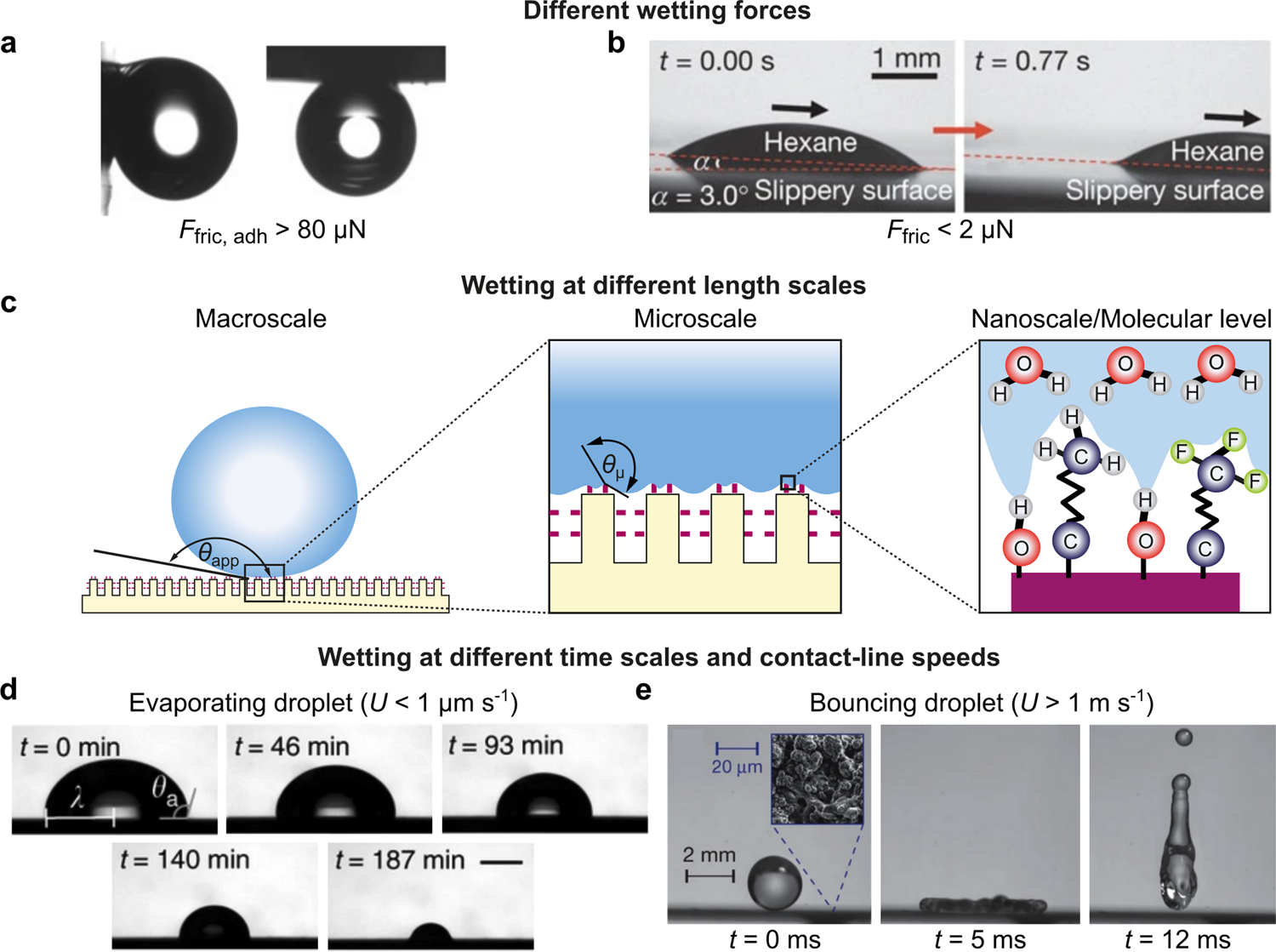
Probing surface wetting across multiple force, length and time
Why do the gas laws only work for gasses above 0 Kelvin (what

822 questions with answers in PHYSICAL CHEMISTRY
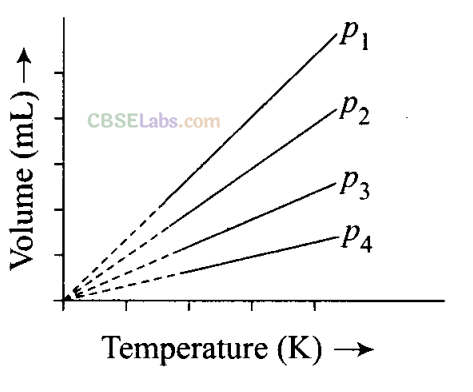
NCERT Exemplar Class 11 Chemistry Chapter 5 States of Matter







